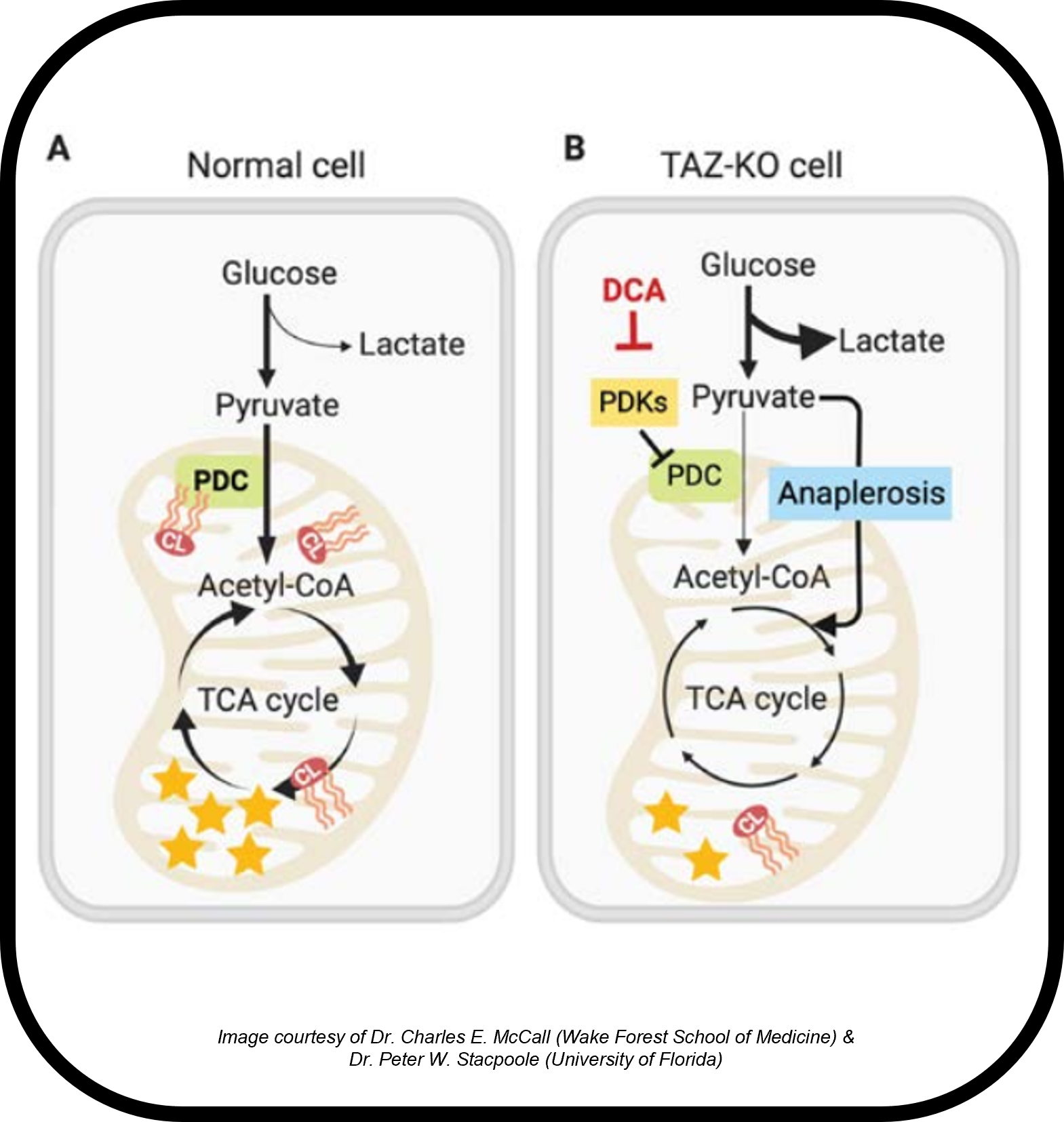
Sepsis causes 270,000 deaths and over $38 billion in healthcare costs each year in the United States, and yet the understanding of its molecular mechanism remains unclear. The dysregulated immune response to infections during sepsis (i.e., shifts from immune resistance to tolerance), has been known to result in bioenergetic perturbations via the disrupted tricarboxylic acid (TCA) cycle. The onset of immunotolerance reduces pyruvate dehydrogenase complex (PDC) activation via increased pyruvate dehydrogenase kinase expression, leading to itaconate-induced inhibition of succinate dehydrogenase by the upregulated itaconate synthesis pathway, which is a catabolic divergence of cis-aconitate anabolism. Similarly, Barth syndrome (BTHS) shares with virtually all primary mitochondrial diseases a fundamental deficiency in cellular energy metabolism. Defective bioenergetics in BTHS exhibits primarily as a life-threatening reduction in myocardial function. Treatment with the prototypic PDC activator dichloroacetate (DCA) has shown promise in decreasing levels of itaconate, potentially reversing metabolic rewiring during sepsis as well as myocardial dysfunction in BTHS. We are using spatial imaging mass spectrometry to determine how DCA treatment reprograms cardiac mitochondrial energetics and, thus, function.
Collaborators
Dr. Peter Stacpoole, UF Division of Endocrinology, Diabetes, and Metabolism – Dr. Stacpoole
Dr. Charles McCall, Wake Forest Molecular Medicine – Dr. McCall
Dr. Lane Smith, Atrium Health Emergency Medicine – Dr. Smith
Current Funding: Barth Syndrome Foundation Development Grant
